New and Revised USP Standards
USP’s Compounding Expert Committee has issued decisions on appeals to recent revisions to General Chapters <795> Pharmaceutical Compounding – Nonsterile Preparations and <797> Pharmaceutical Compounding – Sterile Preparations. USP remains committed to keeping everyone informed of the progress of these standard appeals which were anticipated to become official on December 1, 2019.
Postponing USP <795> and <797>
With the recent postponement of the revised <795> and <797>, there is no immediate impact on current compounding compliance efforts. The postponement is due to appeals around the following topics:
- Beyond-use date provisions for both chapters
- Removal of the alternative technology provision from USP Chapter <797>
- Applicability of both chapters to veterinary practitioners
Though there has been no definitive timeline set, it is suggested that pharmacies operate under the assumption that this is likely to be only a brief delay and be ready to demonstrate full compliance in the coming months.
Due to this delay, the majority of state boards that have already adopted <797> will not move forward with revisions to their rules and regulations until the new chapter is finalized with no pending appeals.
Comparing BUDs Between Official <795> and Revised <795>
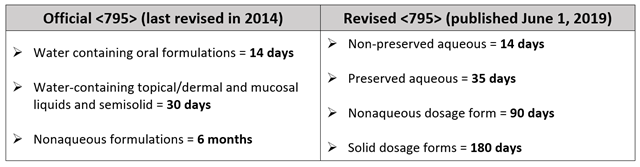 The revised chapter addresses Compounded Nonsterile Preparations (CNSPs) requiring shorter BUDs and BUDs for CNSPs that may be extended.
The revised chapter addresses Compounded Nonsterile Preparations (CNSPs) requiring shorter BUDs and BUDs for CNSPs that may be extended.
The Effects on USP <800>
Even though USP Chapter <800> becomes official on December 1, 2019, it has been stated by USP that it is informational and not fully applicable due to the postponement and pending resolution of the <795> and <797> chapters.
However, some states will be enforcing USP <800> in its current form as of December 1st. In these states, each entity has the option of deciding which version of the chapter they will be inspected under.
Because some states are choosing to enforce all these new chapters, it is important that each site look to their state regulating bodies for guidance.
Sources:
https://www.usp.org/compounding
For more information, visit U.S. Pharmacopoeia's website linked above.
